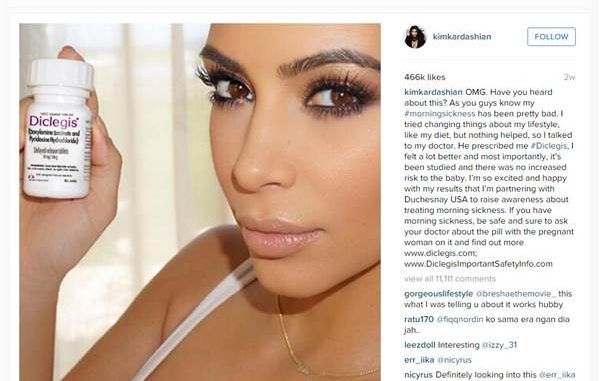
Kim Kardashian is pregnant, which means she is doing product placement – but not without the disapproval of some organizations – like the FDA.
NBC News reports:
An Instagram picture featuring Kardashian, morning sickness drug Diclegis and accompanying text promoting the drug’s benefits garnered more than 450,000 “likes” but brought about a formal FDA warning letter for the drug’s manufacturer.

BYPASS THE CENSORS
Sign up to get unfiltered news delivered straight to your inbox.
You can unsubscribe any time. By subscribing you agree to our Terms of Use

Part of the letter says, “The social media post is misleading because it presents various efficacy claims for Diclegis, but fails to communicate any risk information.”
The posts promoting the drug have since been taken down, but Kardashian, who was paid to promote the drug, originally claimed it made her feel “a lot better and most importantly, it’s been studied and there was no increased risk to the baby.”Duchesnay, the pharmaceutical company behind the drug, told CNBC that while Kim was paid to promote her experience with the drug she had found it on her own accord through her family OB-GYN. The company said it would take quick action in responding to the FDA’s letter.
“Duchesnay USA takes its regulatory responsibilities very seriously, and acknowledges that its communications, including in social media as in this particular instance, need to be in accordance with applicable rules and regulations,” the company said.The FDA warning letter also requests Duchesnay offer corrections to the claims “using the same media” for the same audience. That could mean a revised selfie for Kim’s 42.4 million followers.
Kardashian did not immediately respond to a request for comment from CNBC.
Latest posts by Royce Christyn (see all)
- Irish Slaves – What The History Books Will Never Tell You - November 1, 2017
- Government Op Who Predicted Super Bowl Score Warns Of Nuclear War - February 18, 2017
- Video: Why Voting Doesn’t Change Anything & Democracy Is A Lie - May 7, 2016

Be the first to comment