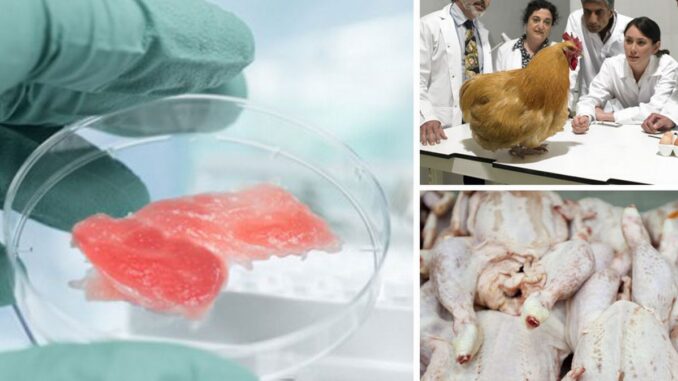
The US government has cleared the way for Americans to be able to eat lab-grown chicken after the Food and Drug Administration declared that chicken created in a laboratory to be safe for human consumption.
Describing the development as “a food revolution,” the FDA claim that the chicken, which is developed by Berkeley, California-based food-tech firm Upside Foods and is produced using animal cell culture technology that takes living cells from chickens and grows the cells in a controlled environment, is safe to eat.

BYPASS THE CENSORS
Sign up to get unfiltered news delivered straight to your inbox.
You can unsubscribe any time. By subscribing you agree to our Terms of Use
Latest Video
The Defender reports: The news — widely reported as an FDA “approval” of lab-grown meat — signifies the completion of the first, and biggest, of the three regulatory steps Upside Foods must complete before its “cultivated” chicken attains full approval and can be sold to the public, according to TIME.
Although two more steps must follow before the FDA can grant the product full approval, the agency’s language suggests the approval is a foregone conclusion.
Upside Foods, on its website, all but confirmed that FDA approval is on the way:
“This landmark regulatory decision means the FDA accepts our safety conclusion, and Upside’s cultivated chicken will be available following USDA inspection and label approval.”
The FDA and some media outlets cheered the news — but others, including scientists and food safety advocates, expressed concerns about the adequacy of the FDA’s preliminary review process.
Experts who spoke to The Defender also questioned the safety of lab-grown meat, which is produced with gene-edited cells, and some scientists argued that, despite claims to the contrary, the production process for lab-grown “meats” is energy-intensive and not, as advertised, beneficial to the environment.
Some also questioned Upside Foods’ connections to figures and entities such as Cargill, Bill Gates, Jeff Bezos, Richard Branson, Kimbal Musk, brother of Elon Musk and co-founder of The Kitchen, “a growing family of businesses that pursues an America where everyone has access to real food,” and the World Economic Forum (WEF).
FDA hasn’t yet granted ‘approval’ — but most significant step in that process completed
The Center for Food Safety said this about the FDA’s announcement:
“The U.S. Food and Drug Administration (FDA) recently completed its preliminary review of the first lab grown ‘chicken’ to be sold as food. The FDA and the U.S. Department of Agriculture are both reviewing ‘meats’ which are grown in vats from cells extracted from living animals.
“This week’s announcement by the FDA that it was reviewing a cell-cultured chicken ‘meat’ is the first indication that these products might come to market in the U.S.”
According to the FDA, this first stage — known as a “pre-market consultation” — was the first time the agency completed such a consultation “for a human food made from cultured animal cells.”
As part of this consultation, according to the FDA:
“The FDA’s pre-market consultation with the firm included an evaluation of the firm’s production process and the cultured cell material made by the production process, including the establishment of cell lines and cell banks, manufacturing controls, and all components and inputs.
“The voluntary pre-market consultation is not an approval process. Instead, it means that after our careful evaluation of the data and information shared by the firm, we have no further questions at this time about the firm’s safety conclusion.”
In an exclusive interview with The Defender, Jaydee Hanson, policy director for the Center for Food Safety, questioned the FDA’s “pre-market consultation”:
“The FDA regulatory process in general relies on company testing of their products.
“The FDA, in this case, seemed to mostly review what the company sent them, but did not require additional tests and did not require the company to disclose its methods in a complete and transparent manner.”
The next steps in the regulatory process involve the U.S. Department of Agriculture and its Food Safety and Inspection Service (FSIS) before full approval is granted.
Upside Foods’ Emeryville, California, production facility will be ready to produce more than 50,000 pounds of “cultivated” products, including a chicken fillet, per year, upon receipt of regulatory approval, the company stated.
Upside Foods, FDA, media describe lab-grown meat as a ‘food revolution,’ ‘watershed moment,’ but regulatory process questioned
The FDA described the news of Upside Foods coming a step closer to full approval as part of “a food revolution” that the world is experiencing, in which the FDA “is committed to supporting innovation in the food supply.”


