
Some conspiracy theorists suspect that NASA are trying to turn Saturn or Jupiter into a small sun in a project dubbed ‘Project Lucifer’.
Disclose.tv reports:

BYPASS THE CENSORS
Sign up to get unfiltered news delivered straight to your inbox.
You can unsubscribe any time. By subscribing you agree to our Terms of Use
Latest Video
If you’re a fan of science fiction, you know about Arthur C. Clarke’s (and Stanley Kubrick’s) seminal novel series 2001: A Space Odyssey.
In the second installment, 2010: Odyssey Two, the alien monolith that was orbiting Jupiter replicates itself and begins condensing the gas giant, eventually transforming it into a smaller sun. This process turns the former planet’s moons into habitable worlds suitable for life.
The people of Earth consequently name the second sun in the sky Lucifer. Without getting too ecclesiastic, the term Lucifer comes from Latin and literally means ‘light-bringing.’
Fitting name for the solar system’s new star. Most of us regard this concept as nothing more than science fiction and nigh impossible to achieve with our current level of technology.
But a number of conspiracy theorists not only believe it possible, they actually claim this is one of NASA’s ongoing projects. Here’s why.
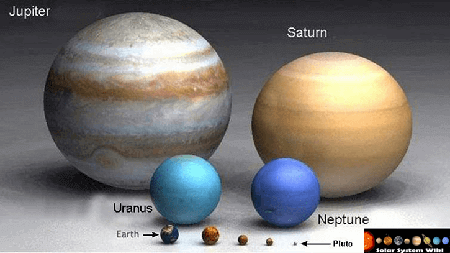
Jupiter has been called a failed star, in the sense that if it had more mass, it could have started nuclear fusion and effectively become a star. Unfortunately (fortunately?), astrophysicists calculated it would have needed to be 75 times more massive in order to do so. Conspiracy theorists believe this mass deficit could be corrected with the addition of nuclear fuel that could jump-start the process of nuclear fusion. Enter NASA’s space probes Galileo, Cassini, Voyager and the rest.
In order for these space probes to work, they need a reliable fuel source as solar energy becomes difficult to catch once you get past the orbit of Mars. Therefore, NASA equipped its probes with an energy source called an RTG, short for radioisotope thermoelectric generator. The radioactive decay of the Plutonium-238 pellets contained in an RTG steadily produces electricity that powers the probe. For example, the Galileo space probe contained 2 RTGs, each carrying 17 pounds of Plutonium-238. What would happen if this payload were detonated inside Jupiter?
Well, nothing, or at least, nothing observable. In 2003, Galileo was deliberately crashed into Jupiter’s atmosphere. NASA followed this approach because they feared crashing it into one of Jupiter’s solid moons carried the risk of contamination with terrestrial bacteria.
Conspiracy theorists saw this venture as an expression of Project Lucifer. They feared that, as Galileo fell through Jupiter’s dense atmosphere, the pressure would have steadily increased, causing the Plutonium in the RTGs to trigger a thermonuclear reaction, birthing the new sun.
It’s been almost twelve years since Galileo was buried in the heart of the gaseous planet and nothing’s happened. There is no second sun on our sky. So we’re in the clear, right?
Not exactly. There’s another gas giant in our solar system: Saturn and it’s not much smaller than Jupiter. And there’s another probe orbiting Saturn, the Cassini space probe. And it’s equipped with 73 pounds of Plutonium-238. And it’s still orbiting Saturn, until commanded otherwise by NASA. So are we in danger?
Physics says no.
Even if every atom of Plutonium aboard the space probe would participate in a nuclear detonation, it wouldn’t be enough to start a chain reaction. It would be like a drop of water in a swimming pool – harmless.
Our technology would need to steadily advance for hundreds if not thousands of years before we could reach the capability of star formation. And even if such technology would be available today, why would we need a second sun? Our own functions perfectly, even with massive UFOs stealing its energy once in a while. If one of the gas giants were to suddenly become a star, it would most likely disrupt the delicate balance of our solar system. Its gravitational pull would tug on the asteroids between Mars and Jupiter, undoubtedly sending some of them our way.
So why did this conspiracy theory gain traction? It must be our fascination with doomsday scenarios.


